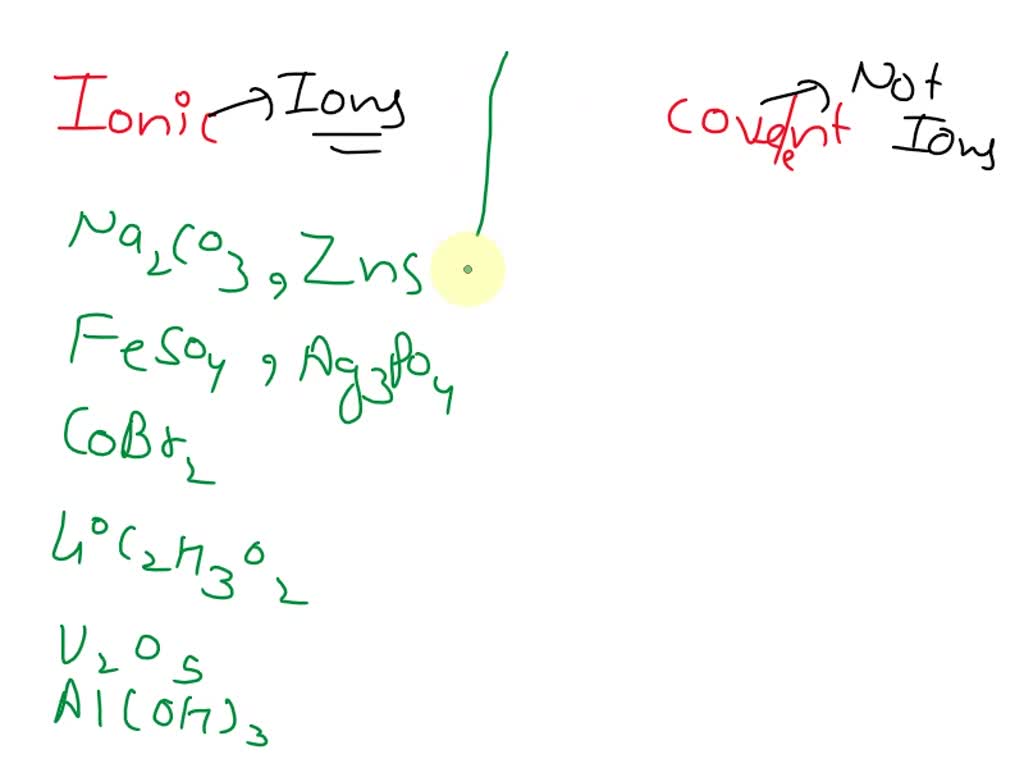Zinc Oxide Ionic Or Covalent . Define ionic and molecular (covalent) compounds. to tell if zno (zinc oxide) is ionic or covalent (also called molecular) we look at. zinc oxide (zno) is an interesting material with respect to conductivity. By the end of this section, you will be able to: That is, does it have ionic bonds, or covalent bonds? It crystallizes in the wurtzite structure, and its bonding is a mix of ionic and. the first question we ask is if the compound is ionic or covalent? covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located. covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of.
from www.numerade.com
to tell if zno (zinc oxide) is ionic or covalent (also called molecular) we look at. It crystallizes in the wurtzite structure, and its bonding is a mix of ionic and. covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located. That is, does it have ionic bonds, or covalent bonds? covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of. the first question we ask is if the compound is ionic or covalent? Define ionic and molecular (covalent) compounds. zinc oxide (zno) is an interesting material with respect to conductivity. By the end of this section, you will be able to:
SOLVED Mixed Ionic/Covalent Compound Naming For each of the following
Zinc Oxide Ionic Or Covalent covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located. covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of. Define ionic and molecular (covalent) compounds. zinc oxide (zno) is an interesting material with respect to conductivity. the first question we ask is if the compound is ionic or covalent? That is, does it have ionic bonds, or covalent bonds? By the end of this section, you will be able to: It crystallizes in the wurtzite structure, and its bonding is a mix of ionic and. covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located. to tell if zno (zinc oxide) is ionic or covalent (also called molecular) we look at.
From www.youtube.com
Is ZnO (Zinc oxide) Ionic or Covalent? YouTube Zinc Oxide Ionic Or Covalent zinc oxide (zno) is an interesting material with respect to conductivity. It crystallizes in the wurtzite structure, and its bonding is a mix of ionic and. to tell if zno (zinc oxide) is ionic or covalent (also called molecular) we look at. That is, does it have ionic bonds, or covalent bonds? By the end of this section,. Zinc Oxide Ionic Or Covalent.
From www.youtube.com
Is ZnF2 Ionic or Covalent/Molecular? YouTube Zinc Oxide Ionic Or Covalent covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located. By the end of this section, you will be able to: It crystallizes in the wurtzite structure, and its bonding is a mix of ionic and. That is, does it have ionic bonds, or. Zinc Oxide Ionic Or Covalent.
From www.researchgate.net
Schematic representation of formation of both ionic and covalent Zinc Oxide Ionic Or Covalent By the end of this section, you will be able to: the first question we ask is if the compound is ionic or covalent? covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of. zinc oxide (zno) is an interesting material with respect to conductivity. . Zinc Oxide Ionic Or Covalent.
From www.numerade.com
SOLVED What type of bonding is used in the formation of Zinc Oxide Zinc Oxide Ionic Or Covalent Define ionic and molecular (covalent) compounds. covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of. covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located. the first question we ask. Zinc Oxide Ionic Or Covalent.
From sciencetrends.com
What Is The Ionic Charge Of Zinc (Zn)? Science Trends Zinc Oxide Ionic Or Covalent That is, does it have ionic bonds, or covalent bonds? By the end of this section, you will be able to: It crystallizes in the wurtzite structure, and its bonding is a mix of ionic and. zinc oxide (zno) is an interesting material with respect to conductivity. covalent bonds are the attractive forces between the positively charged nuclei. Zinc Oxide Ionic Or Covalent.
From www.dreamstime.com
Zinc Oxide is a Molecular Chemical Formula. Zinc Infographics. Vector Zinc Oxide Ionic Or Covalent to tell if zno (zinc oxide) is ionic or covalent (also called molecular) we look at. It crystallizes in the wurtzite structure, and its bonding is a mix of ionic and. the first question we ask is if the compound is ionic or covalent? Define ionic and molecular (covalent) compounds. covalent bonds are the attractive forces between. Zinc Oxide Ionic Or Covalent.
From studylib.net
Ionic/Covalent Compound Naming Solutions Zinc Oxide Ionic Or Covalent By the end of this section, you will be able to: covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of. covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located. That. Zinc Oxide Ionic Or Covalent.
From en.wikipedia.org
Ionic bonding Wikipedia Zinc Oxide Ionic Or Covalent covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located. Define ionic and molecular (covalent) compounds. By the end of this section, you will be able to: zinc oxide (zno) is an interesting material with respect to conductivity. covalent bonds are the. Zinc Oxide Ionic Or Covalent.
From www.pinterest.com
Difference Between Covalent and Ionic Bonds Chemistry education Zinc Oxide Ionic Or Covalent Define ionic and molecular (covalent) compounds. That is, does it have ionic bonds, or covalent bonds? the first question we ask is if the compound is ionic or covalent? zinc oxide (zno) is an interesting material with respect to conductivity. to tell if zno (zinc oxide) is ionic or covalent (also called molecular) we look at. By. Zinc Oxide Ionic Or Covalent.
From www.dreamstime.com
Zinc Oxide Chemical Formula. Vector Illustration. Stock Image Stock Zinc Oxide Ionic Or Covalent covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located. It crystallizes in the wurtzite structure, and its bonding is a mix of ionic and. That is, does it have ionic bonds, or covalent bonds? By the end of this section, you will be. Zinc Oxide Ionic Or Covalent.
From www.numerade.com
SOLVED 'Identify the ionic and covalent bond in the following Zinc Oxide Ionic Or Covalent covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of. zinc oxide (zno) is an interesting material with respect to conductivity. covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located.. Zinc Oxide Ionic Or Covalent.
From www.thesciencehive.co.uk
Ionic Bonding — the science sauce Zinc Oxide Ionic Or Covalent That is, does it have ionic bonds, or covalent bonds? to tell if zno (zinc oxide) is ionic or covalent (also called molecular) we look at. the first question we ask is if the compound is ionic or covalent? Define ionic and molecular (covalent) compounds. It crystallizes in the wurtzite structure, and its bonding is a mix of. Zinc Oxide Ionic Or Covalent.
From stock.adobe.com
Zinc oxide, ZnO molecule. It is compound, mineral ingredient Zinc Oxide Ionic Or Covalent the first question we ask is if the compound is ionic or covalent? covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of. zinc oxide (zno) is an interesting material with respect to conductivity. covalent bonds are the attractive forces between the positively charged nuclei. Zinc Oxide Ionic Or Covalent.
From www.britannica.com
Ionic bond Definition, Properties, Examples, & Facts Britannica Zinc Oxide Ionic Or Covalent It crystallizes in the wurtzite structure, and its bonding is a mix of ionic and. zinc oxide (zno) is an interesting material with respect to conductivity. Define ionic and molecular (covalent) compounds. to tell if zno (zinc oxide) is ionic or covalent (also called molecular) we look at. the first question we ask is if the compound. Zinc Oxide Ionic Or Covalent.
From exoplzjjb.blob.core.windows.net
Ionic Bond Definition Vs Covalent at Dennis Wells blog Zinc Oxide Ionic Or Covalent By the end of this section, you will be able to: covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located. Define ionic and molecular (covalent) compounds. zinc oxide (zno) is an interesting material with respect to conductivity. to tell if zno. Zinc Oxide Ionic Or Covalent.
From testbook.com
Zinc Oxide Learn Definition, Formula, Preparation and Reactions Zinc Oxide Ionic Or Covalent covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located. By the end of this section, you will be able to: covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of. . Zinc Oxide Ionic Or Covalent.
From wisc.pb.unizin.org
M11Q3 Types of Solids Chem 103/104 Resource Book Zinc Oxide Ionic Or Covalent to tell if zno (zinc oxide) is ionic or covalent (also called molecular) we look at. Define ionic and molecular (covalent) compounds. covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located. By the end of this section, you will be able to:. Zinc Oxide Ionic Or Covalent.
From www.youtube.com
How to Write the Net Ionic Equation for Zn + CH3COOH = (CH3COO)2Zn + H2 Zinc Oxide Ionic Or Covalent covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located. By the end of this section, you will be able to: to tell if zno (zinc oxide) is ionic or covalent (also called molecular) we look at. It crystallizes in the wurtzite structure,. Zinc Oxide Ionic Or Covalent.